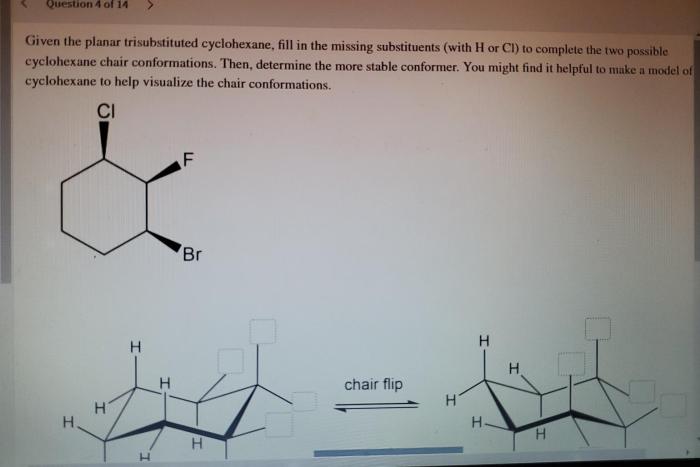
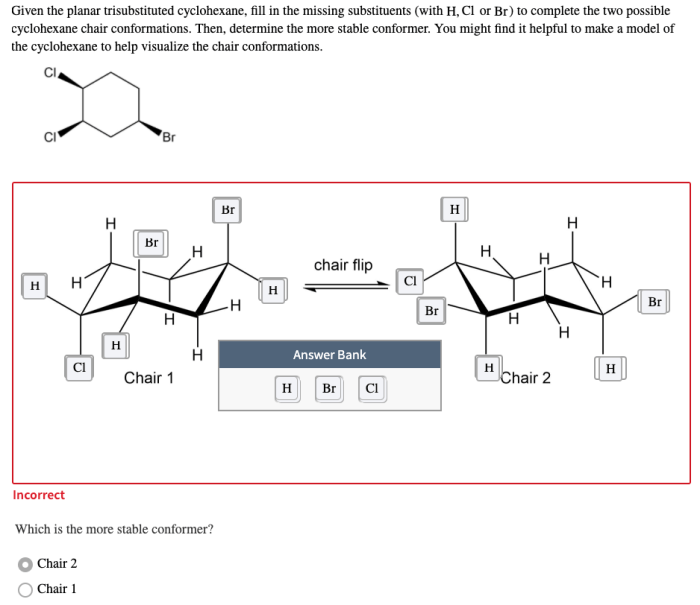
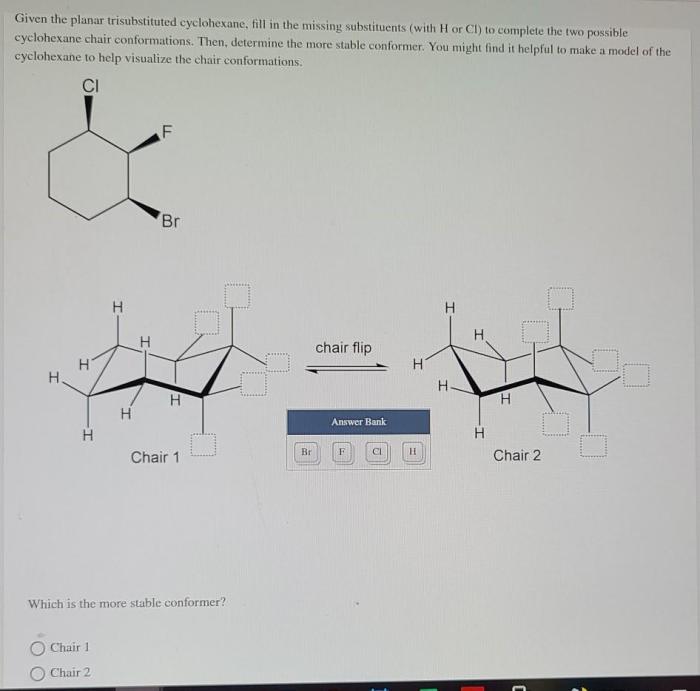
Given the planar trisubstituted cyclohexane fill in the missing substituents – Given the planar trisubstituted cyclohexane: Filling in the Missing Substituents, this article delves into the intricate world of organic chemistry, exploring the structural nuances and conformational dynamics of a specific class of cyclohexane derivatives. By examining the interplay between substituents and planarity, we uncover the factors that govern the molecular properties and applications of these versatile compounds.
Planar trisubstituted cyclohexanes, characterized by their unique structural features and conformational flexibility, offer a rich landscape for scientific inquiry. Their significance extends to diverse fields, including pharmaceuticals, materials science, and beyond. This article aims to provide a comprehensive understanding of these compounds, shedding light on their properties, applications, and potential future advancements.
Structural Analysis of Planar Trisubstituted Cyclohexane: Given The Planar Trisubstituted Cyclohexane Fill In The Missing Substituents
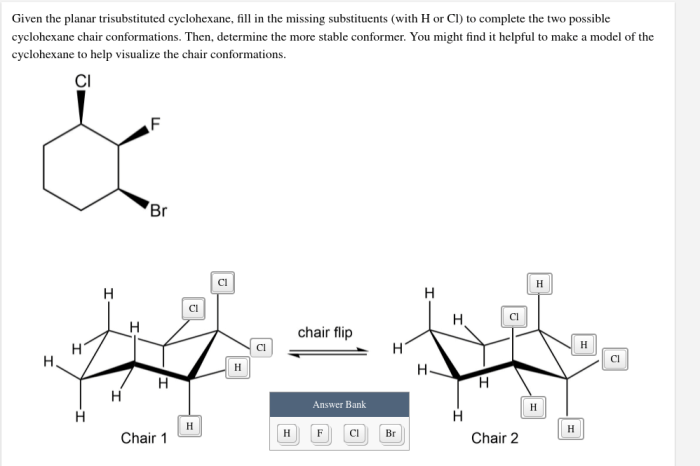
Planar trisubstituted cyclohexane is a unique structural isomer of cyclohexane characterized by the presence of three substituents attached to the same carbon atom in the ring. This arrangement results in a non-planar ring conformation, with the three substituents occupying the same plane.
The planar configuration of trisubstituted cyclohexane has significant implications for its molecular properties. It influences the molecule’s stability, reactivity, and interactions with other molecules.
Substituent Effects on Planarity
The nature and position of substituents play a crucial role in determining the planarity of the cyclohexane ring. Electron-withdrawing substituents, such as fluorine or chlorine, tend to promote planarity by reducing the steric hindrance between the substituents.
Conversely, bulky substituents, such as methyl or tert-butyl groups, hinder planarity by increasing the steric interactions between the substituents. The size, shape, and electronic properties of the substituents must be carefully considered when predicting the planarity of trisubstituted cyclohexanes.
Conformational Analysis
Planar trisubstituted cyclohexanes can adopt different conformations, each with its own unique energy profile. The relative stability of these conformations depends on the steric and electronic interactions between the substituents.
For example, the most stable conformation of 1,3,5-trimethylcyclohexane is the chair conformation, in which the three methyl groups are oriented in an equatorial position to minimize steric hindrance.
Applications of Planar Trisubstituted Cyclohexanes, Given the planar trisubstituted cyclohexane fill in the missing substituents
Planar trisubstituted cyclohexanes find applications in various fields, including pharmaceuticals, materials science, and catalysis.
In the pharmaceutical industry, planar trisubstituted cyclohexanes are used as building blocks for the synthesis of complex drug molecules. Their unique structural properties make them suitable for designing drugs with specific biological activities.
Quick FAQs
What is the significance of planarity in trisubstituted cyclohexanes?
Planarity in trisubstituted cyclohexanes influences their molecular properties, including reactivity, stability, and conformational preferences. It affects the interactions between substituents and the cyclohexane ring, leading to unique structural and electronic characteristics.
How do substituents affect the planarity of cyclohexane rings?
Substituents can promote or hinder planarity depending on their steric and electronic properties. Bulky substituents can induce non-planarity due to steric hindrance, while electron-withdrawing substituents can enhance planarity by reducing ring strain.
What are the different conformations that planar trisubstituted cyclohexanes can adopt?
Planar trisubstituted cyclohexanes can adopt various conformations, including chair, boat, and twist-boat conformations. The relative stability of these conformations depends on the nature and position of the substituents, as well as the energy barriers associated with conformational interconversions.